Empowering Better Sight - bringing science, compliance, & innovation into perfect focus.
Maintaining an effective and industry-leading Quality Management System to ensure every product reflects our dedication to innovation and precision..
Laurus Optics Limited is a leading Hong Kong-based manufacturer specializing in the design, production, quality control, and sales of high-quality intraocular lenses and ophthalmic medical devices.
At Laurus Optics, we combine passion with precision to deliver high-quality ophthalmic products that meet the highest international standards. Our robust Quality Management System is certified under ISO 13485:2016, aligned with EU Medical Device Regulations (MDR) and other global requirements.
We offer a comprehensive portfolio of ophthalmic solutions, including trusted intraocular lens brands such as Acuity, Attos, Clauris, Lauralens, and Sequor. Through close collaboration with our customers, we remain committed to innovation and advancing ophthalmic technologies worldwide.
Customer-Centric Approach: Products Designed Around Those Who Rely on Them

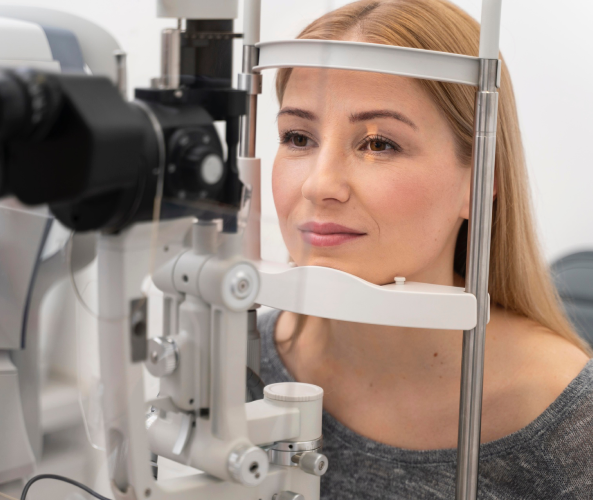
Customer-Centric Approach: Products Designed Around Those Who Rely on Them
Collaboration is at the heart of everything we do. By fostering strong relationships with our customers, Laurus Optics continually advances products and technologies to address evolving market demands. Our dedication to innovation ensures that we remain at the forefront of vision care solutions.
Accreditations & Standards
ISO 13485:2016
Laurus Optics is ISO 13485:2016 certified, in accordance with EU Medical Device Regulations (MDR) and other global standards. This certification reflects our ongoing commitment to quality, compliance, and patient safety in every product we deliver.
China NMPA
Laurus Optics is certified by the China National Medical Products Administration (NMPA) for intraocular lenses and surgical tools. These approvals underscore our dedication to meeting regulatory standards and ensuring safe, high-quality solutions for the Chinese market.
Conformité Européenne
Laurus Optics holds CE certification in compliance with European safety, health, and environmental protection standards. This mark affirms our commitment to delivering high-performance ophthalmic devices that meet the needs of surgeons and patients across global markets.
01
ISO 13485:2016
Laurus Optics is ISO 13485:2016 certified, in accordance with EU Medical Device Regulations (MDR) and other global standards. This certification reflects our ongoing commitment to quality, compliance, and patient safety in every product we deliver.
02
China NMPA
Laurus Optics is certified by the China National Medical Products Administration (NMPA) for intraocular lenses and surgical tools. These approvals underscore our dedication to meeting regulatory standards and ensuring safe, high-quality solutions for the Chinese market.
03
Conformité Européenne
Laurus Optics holds CE certification in compliance with European safety, health, and environmental protection standards. This mark affirms our commitment to delivering high-performance ophthalmic devices that meet the needs of surgeons and patients across global markets.