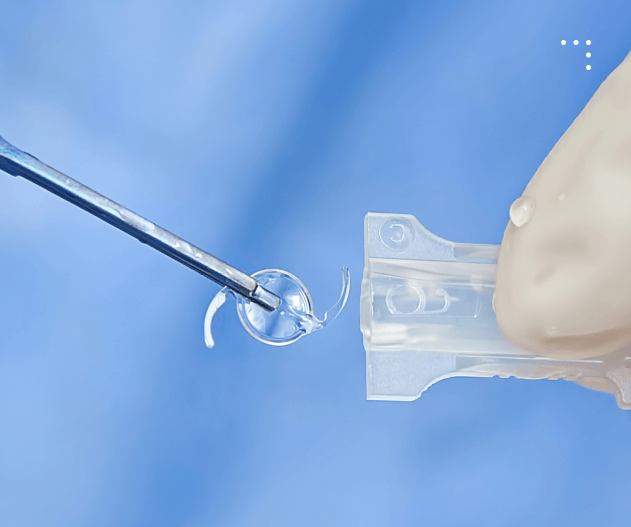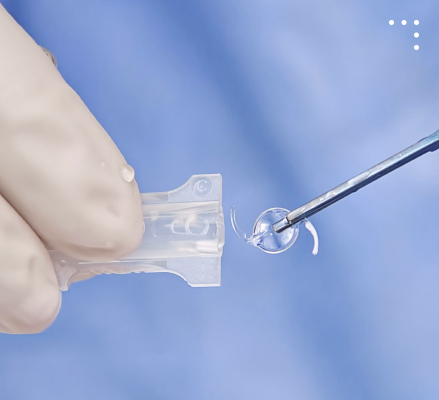







Meeting the needs of Ophthalmic Surgeons Our Products
Single Use
Injector Systems
Sterile, disposable device designed for safe, precise delivery of intraocular lenses during eye surgery.
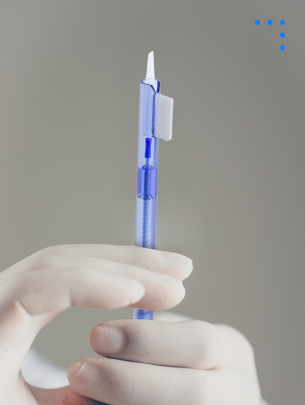
Preloaded
Injector Systems
intraocular lens preloaded in a sterile, disposable device designed for safe and precise delivery during eye surgery.
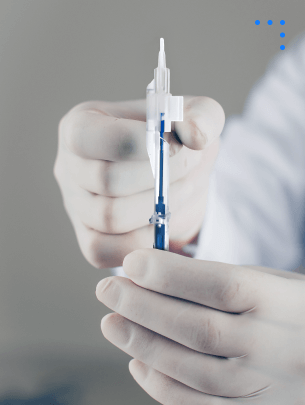
Non Absorbable Nylon Suture With Needles
Strong, sterile sutures for precise, long-term wound closure.

Ophthalmic
Surgical Blades
Ultra-sharp, sterile blades for precise, reliable eye surgery.

Meeting the needs of Ophthalmic Surgeons Our Products
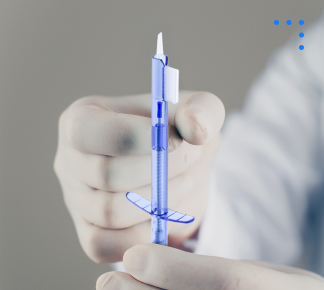
Single Use Injector Systems
Sterile, disposable device designed for safe, precise delivery of intraocular lenses during eye surgery.

Ophthalmic Surgical Blades
Ultra-sharp, sterile blades for precise, reliable eye surgery.

Non Absorbable Nylon Suture With Needles
Strong, sterile sutures for precise, long-term wound closure.
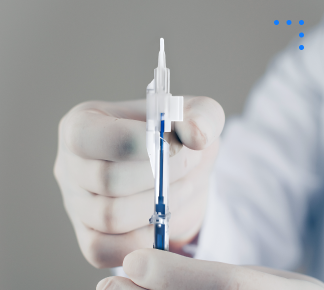
Preloaded Injector Systems
Sterile, disposable device designed for safe, precise delivery of intraocular lenses during eye surgery.
Meeting the needs of Ophthalmic Surgeons Our Products
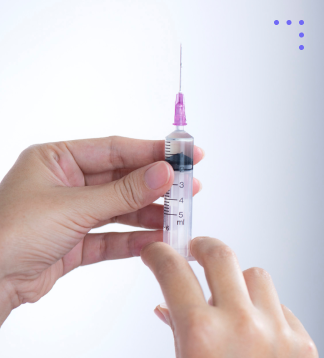
Single Use Injector Systems
The Latest Announcements, Events & Milestones

Key Departments Driving Innovation
01
Research and Development
Our R&D department innovates intraocular lenses (IOLs) to enhance visual clarity and patient outcomes in cataract surgery using advanced materials and optical engineering.

02
Manufacturing
Our Manufacturing department produces high-quality intraocular lenses (IOLs) with state-of-the-art facilities, ensuring precision, consistency, safety, and scalability to meet market demand for reliable eye care products.

03
Engineering
The Engineering department at Laurus Optics Ltd. designs advanced intraocular lenses (IOLs), combining optics, materials science, and biomechanics for superior performance and durability in surgery.
04
Supply Chain
Laurus Optics Ltd.’s Supply Chain department handles materials, components, and finished products, focusing on strategic sourcing, efficient logistics, and inventory optimization for reliable, cost-effective operations.
05
Quality and Regulatory
The Quality and Regulatory department ensures intraocular lenses (IOLs) meet high safety standards via rigorous testing, audits, and compliance with ISO 13485 and EU regulations for excellence.

Key Departments Driving Innovation
Research and Development
Our Research and Development department innovates intraocular lenses (IOLs) to enhance visual clarity and patient outcomes in cataract surgery using advanced materials and optical engineering.
Manufacturing and Production
Our Manufacturing department produces premium intraocular lenses (IOLs) in state-of-the-art facilities, ensuring precision, safety, consistency, and scalability to meet the growing demand for reliable eye care products.
Engineering and Design
Our Engineering department designs high-performance intraocular lenses (IOLs), combining optics, material science, and biomechanics to ensure durability and superior surgical performance.
Supply Chain Management
Out Supply Chain department manages raw materials, components, and finished IOLs, focusing on strategic sourcing, efficient logistics, and inventory optimization for reliable, cost-effective operations.
Quality and Regulatory
Our Quality and Regulatory department ensures that all intraocular lenses (IOLs) meet global safety standards through rigorous testing, internal audits, and strict compliance with ISO 13485 and EU medical regulations.